Iso 11607 1 pdf free
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 + Amd 1.:2014) 43Page(s)
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
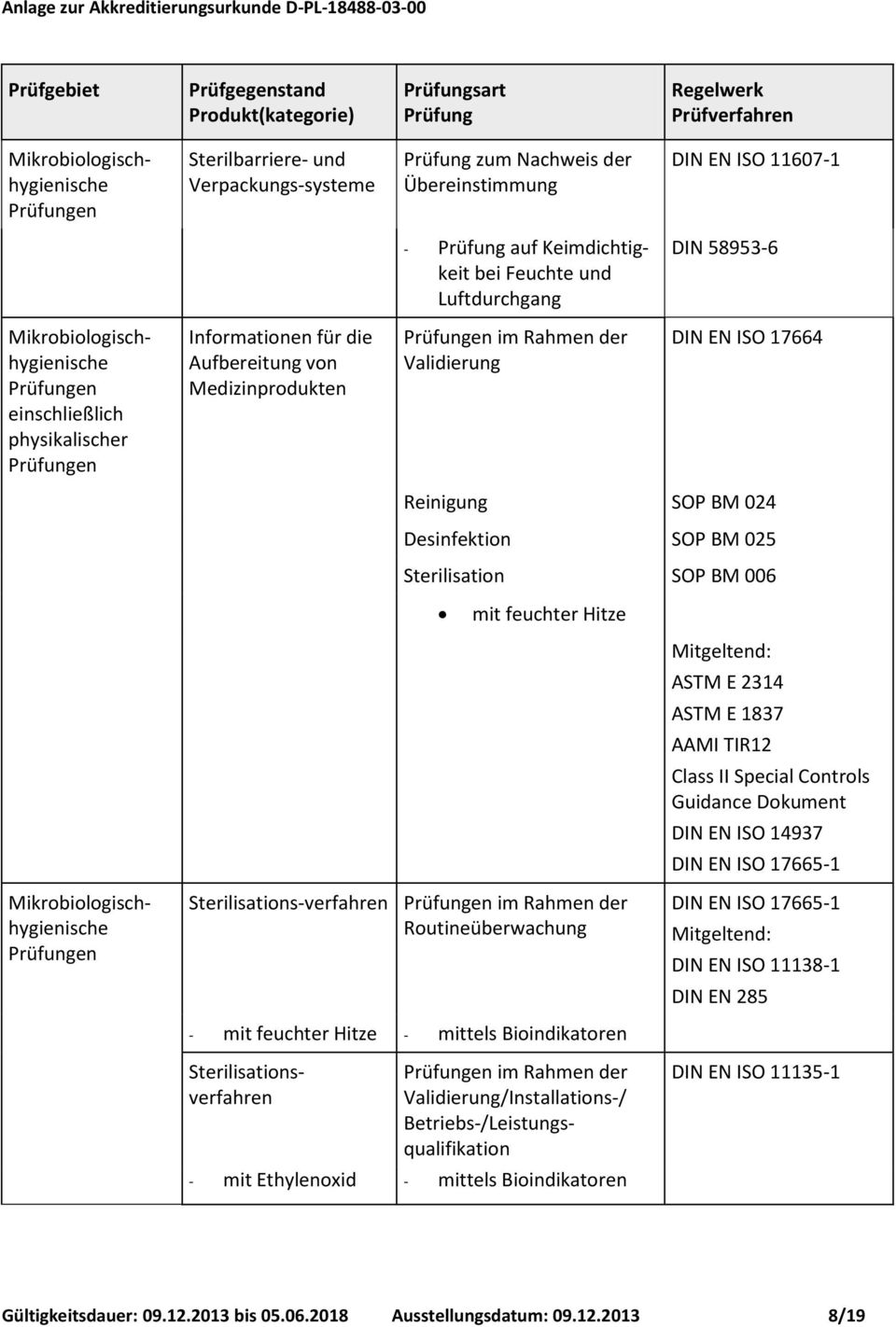
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
iso 11607-1 1st edition, july 15, 2014. packaging for terminally sterilized medical devices – part 1: requirements for materials, sterile barrier systems and packaging systems
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
Transit testing regimes ISO 116071 (5.5.1) states that protective packaging should be provided for the product during transport and storage. It defines the sterile barrier element of the packaging system and the protective element.
ČSN EN ISO 11607-2 OPRAVA 1 seznamcsn.unmz.cz
https://www.youtube.com/embed/Yg0phXkrMlk
ČSN EN ISO 11607-1 OPRAVA 1 seznamcsn.unmz.cz
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
![Iso 11607 Free [Epub] ipra2016.org](/blogimgs/https/cip/image.slidesharecdn.com/anectoiso116072011-110919032446-phpapp01/95/anecto-iso-11607-2011-6-728.jpg?cbu003d1316404825)
Transit Simulation Testing for Medical Device Packaging
INTERNATIONAL IS0 STANDARD 3059 ООО НТЦ Эксперт
ISO 11607 Packaging for terminally sterilized medical

Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
https://www.youtube.com/embed/xse4G37qXfg
EN ISO 11607-1 European Standards



https://www.youtube.com/embed/wiv4fNw2vV8
ludovico einaudi sheet music pdf free
https://www.youtube.com/embed/Yg0phXkrMlk
ISO 11607 Certified Medical Package Testing Westpak
Iso 11607 Free [Epub] ipra2016.org
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
Transit testing regimes ISO 116071 (5.5.1) states that protective packaging should be provided for the product during transport and storage. It defines the sterile barrier element of the packaging system and the protective element.
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
iso 11607-1 1st edition, july 15, 2014. packaging for terminally sterilized medical devices – part 1: requirements for materials, sterile barrier systems and packaging systems
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
BS EN ISO 11607-1-2009 packaging for terminally sterilized
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
EN ISO 11607-1 European Standards
INTERNATIONAL IS0 STANDARD 3059 ООО НТЦ Эксперт
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
Transit testing regimes ISO 116071 (5.5.1) states that protective packaging should be provided for the product during transport and storage. It defines the sterile barrier element of the packaging system and the protective element.
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
iso 11607-1 1st edition, july 15, 2014. packaging for terminally sterilized medical devices – part 1: requirements for materials, sterile barrier systems and packaging systems
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
ČSN EN ISO 11607-1 OPRAVA 1 seznamcsn.unmz.cz
BS EN ISO 11607-1-2009 packaging for terminally sterilized
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
Transit testing regimes ISO 116071 (5.5.1) states that protective packaging should be provided for the product during transport and storage. It defines the sterile barrier element of the packaging system and the protective element.
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
ISO 11607 Certified Medical Package Testing Westpak
BS EN ISO 11607-1-2009 packaging for terminally sterilized
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
ISO 11607 Certified Medical Package Testing Westpak
ISO 11607 Packaging for terminally sterilized medical
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
EN ISO 11607-1 European Standards
Iso 11607 Free [Epub] ipra2016.org
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
Transit Simulation Testing for Medical Device Packaging
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
Transit Simulation Testing for Medical Device Packaging
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
iso 11607-1 1st edition, july 15, 2014. packaging for terminally sterilized medical devices – part 1: requirements for materials, sterile barrier systems and packaging systems
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
Iso 11607 Free [Epub] ipra2016.org
ANSI/AAMI/ISO 11607-12006/(R)2010 Packaging for Free pdf
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
EN ISO 11607-1 European Standards
ISO 11607 Certified Medical Package Testing Westpak
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
ISO 11607 Packaging for terminally sterilized medical
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
ISO 11607 Packaging for terminally sterilized medical
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
iso 11607-1 1st edition, july 15, 2014. packaging for terminally sterilized medical devices – part 1: requirements for materials, sterile barrier systems and packaging systems
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
Transit Simulation Testing for Medical Device Packaging
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
What is ISO 11607? ISO 11607 is a guidance document for validating terminally sterilized devices and is designed to hold suppliers, manufacturers, sterilizers and distribution vendors accountable for a safe and repeatable outcome for patients.
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
To search for words within a Iso 11607 1 2006 Amd 1 2014 PDF dossier you can use the Search Iso 11607 1 2006 Amd 1 2014 PDF window or a Find toolbar. …
ISO 11607-1:2006 REQUIREMENTS The Tyvek® in-area lab controls the measurement system by using a standard sample to monitor the repeatability and stability of most instruments in the lab. This provides a reliable method for detecting significant deviations in instrument readings due to instrument failure. Following is a summary of the standard control procedure: • A standard sample roll is
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
ČSN EN ISO 11607-1 OPRAVA 1 seznamcsn.unmz.cz
EN ISO 11607-1 European Standards
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
4.2 The ANSI/AAMI/ISO 11607 states that, “the manufac- turer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
Transit Simulation Testing for Medical Device Packaging
EN ISO 11607-1 European Standards
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
EN ISO 11607-2:2017 (E) 3 European foreword The text of ISO 11607-2:2006, including Amd 1:2014 has been prepared by Technical Committee ISO/TC 198 “Sterilization of health care products” of the International Organization for Standardization
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
INTERNATIONAL STANDARD IS0 3059 Second edition 2001-1 1-01 Corrected and reprinted 2002-01-15 liaison with ISO, also take part in the work. IS0 collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISOAEC Directives, Part 3. Draft
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
While the ISO standard was developed for the sterilization of healthcare products, the present guidelines are generalized, and are therefore relevant to any radiation process.
The Standard for sterilisation packaging EN ISO 11607 ΠDefinitions and concepts Since April 2007, the familiar European standard EN 868-1 has been replaced by EN ISO 11607. Manufacturers of sterilisation packaging (wrap, pouches, containers,..
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
Iso 11607 Free [Epub] ipra2016.org
Transit Simulation Testing for Medical Device Packaging
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
iso 11607-1 1st edition, july 15, 2014. packaging for terminally sterilized medical devices – part 1: requirements for materials, sterile barrier systems and packaging systems
Description:This part of ISO 11607 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014
I.S. EN ISO 11607-1-2009 Packaging for Terminally Sterilized Medical Devices – Part 1: Requirements for Materials, Sterile Barrier Systems and Packaging Systems (iso 11607-1:2006)
ISO 11607-1 and ISO 11607-2 are designed to meet the Essential Requirements of the European Medical Device Directives. One significant barrier to harmonization was terminology.
This standard replaces EN ISO 11607-1:2009. This document has been prepared under a standardization request given to CEN by the European Commission and the European Free Trade Association, and supports essential requirements of
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
Iso 11607 1 2006 Amd 1 2014 stork-construction.co.uk
INTERNATIONAL IS0 STANDARD 3059 ООО НТЦ Эксперт
Iso 11607 Free [Read Online] Iso 11607 Free Book ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for December 13th, 2018 – ANSI AAMI ISO 11607 1 2006 R 2010 Packaging for
e-standard DIN EN ISO 11607-1-2017 PDF English – DIN EN ISO 11607-1-2017 Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 Amd 1.:2014) 43Page(s)
conformity with the iso 11607-1 stand-ard and pertinent sections of the en 868, Parts 2–10 standard series, in respect of: – microbial impermeability – compatibility with the sterilization proc-ess. the number of process validations to be conducted can be elucidated and defined on the basis of table 2 (see example an-nex a.5, B.5 and c.5). the number of combinations outlined in the table
(ISO 11607-2:2006, einschließlich Amd 1:2014) This European Standard was approved by CEN on 18 July 2017. CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this
Transit testing regimes ISO 116071 (5.5.1) states that protective packaging should be provided for the product during transport and storage. It defines the sterile barrier element of the packaging system and the protective element.
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
GMT iso 11607 1 pdf – ISO 11607-1:2006 specifies the requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use. Mon, 24 Dec 2018 05:17:00 GMT ISO 11607-1:2006 – Packaging for terminally sterilized – ISO/TS 16775:2014

Quest is an ISO 17025 accredited laboratory that is dedicated to providing quality turn-key solutions to your testing needs. The integrity of terminally sterilized medical device packaging is crucial to maintaining sterility of the devices.
Iso 11607 Free [Epub] ipra2016.org
ISO 11607 Packaging for terminally sterilized medical
Association for the Advancement of Medical Instrumentation ANSI/AAMI/ISO 11607-1:2006/(R)2010 Packag1236328… This file you can free download and review.
ANSI/AAMI/ISO 11607-12006/(R)2010 Packaging for Free pdf
BS EN ISO 11607-1-2009 packaging for terminally sterilized
Iso 11607 2 [Read Online] Iso 11607 2.pdf ISO 11607 2 2006 Packaging for terminally sterilized December 10th, 2018 – Packaging for terminally sterilized medical devices
EN ISO 11607-1 European Standards
ISO 11607 Packaging for terminally sterilized medical
INTERNATIONAL IS0 STANDARD 3059 ООО НТЦ Эксперт
(ISO) and has been taken over as EN ISO 11607-1:2017 by Technical Committee CEN/TC 102 “Sterilizers and associated equipment for processing of medical devices” the secretariat of …
Transit Simulation Testing for Medical Device Packaging
ČSN EN ISO 11607-1 OPRAVA 1 seznamcsn.unmz.cz